Allylic and vinylic imidation of unactivated alkenes using n fluorobenzenesulfonimide nfsi as both the terminal oxidant and the nitrogen source is described.
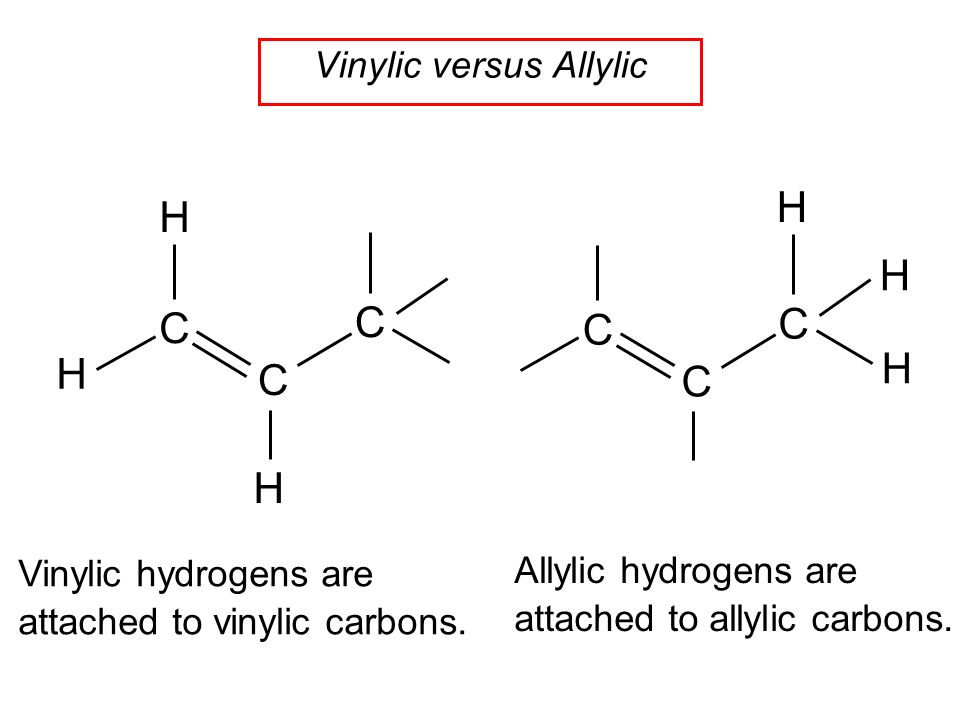
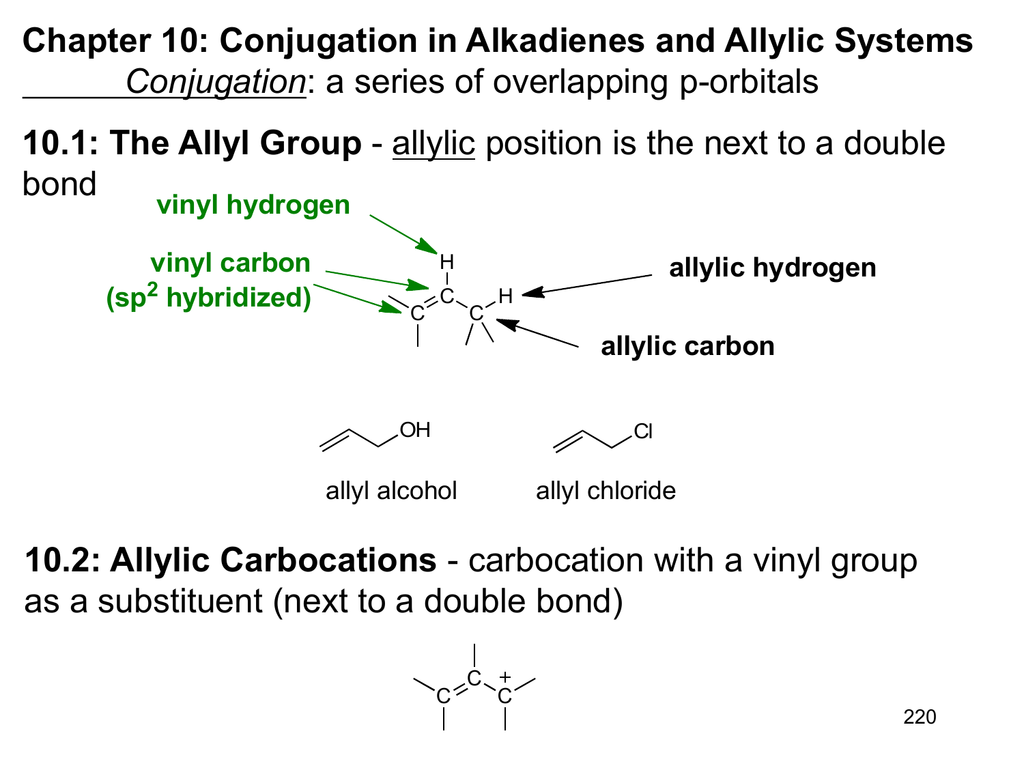
Substitution at allylic and vinylic position of alkenes.
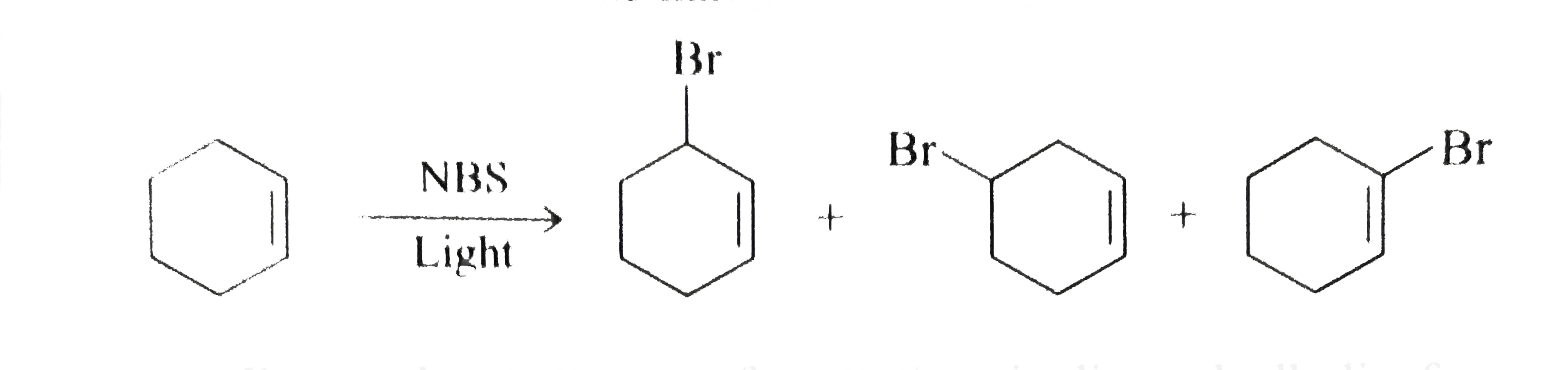
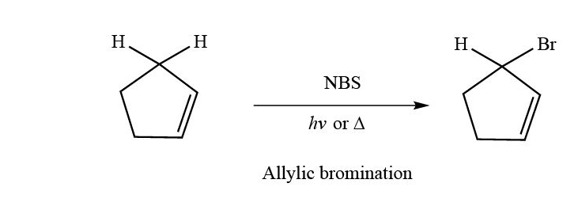
The allylic substitution is very selective and is followed when the desired product is required to possess double bond character as well as a reactive site such as allylic position as bromide is a good leaving group which can be substituted by another nucleophilic species.
Radical additions and substitutions with alkenes radical bromination in allylic position the addition of halogens to alkenes at room temperature with a high halogen concentration yields vicinal dihalogenides.
At low temperatures and a high halogen concentration an addition to the double bond occurs.
It was also demonstrated that vinylimides formed by this procedure can be used as masked enamides through chemoselective cleavage of a sulfonyl group.
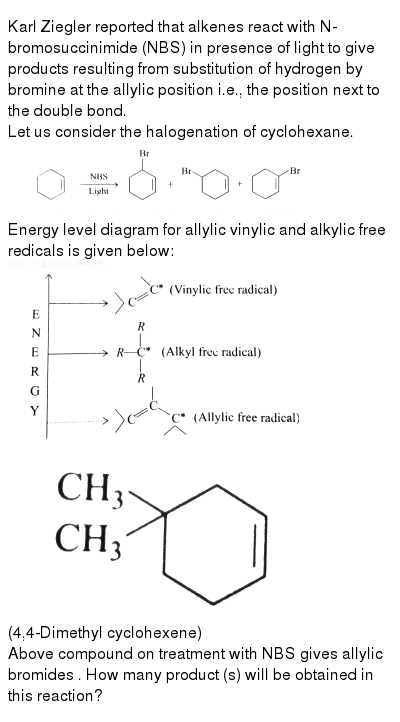
Why substitution of allylic hydrogens.
It s easy to record your screen and livestream.
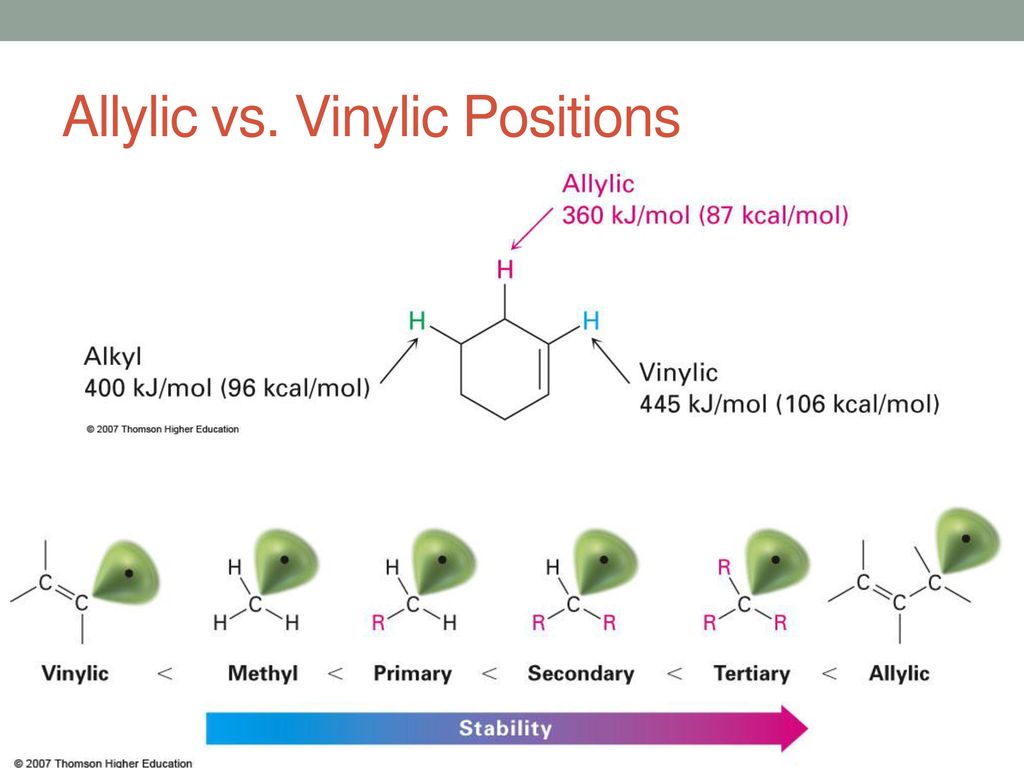
As the table below shows the dissociation energy for the allylic c h bond is lower than the dissociation energies for the c h bonds at the vinylic and alkylic positions.
Depending on the conditions the reactions of propene with either bromine or chlorine respectively yields different products.
In contrast a substitution of hydrogen at the methyl group is common among high temperatures and a low halogen concentration.
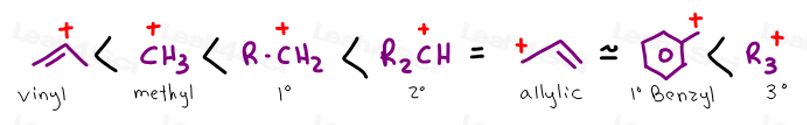
This is because the radical formed when the allylic hydrogen is removed is resonance stabilized.
Radical substitution in allylic position.



.jpg?revision=1)